Background: Patients with Diffuse Large B-Cell Lymphoma (DLBCL) who are treated in low-resource settings have inferior outcomes compared to those in high-resource settings, with 12-month overall survival (OS) rates typically under 50% with standard chemotherapy (Gopal et al. [PMID:26934054]). Rituximab, an anti-CD20 monoclonal antibody, when combined with chemotherapy, improves OS for DLBCL and is the standard of care for patients with CD20+ DLBCL in resource-rich settings. Despite receiving FDA approval over 25 years ago and its subsequent inclusion in the World Health Organization's List of Essential Medicines, IV rituximab is not readily available or routinely used in sub-Saharan Africa (SSA). This is in part due to cost and the limited availability of infusion centers in SSA. Subcutaneous rituximab hyaluronidase (sqR) is a potential solution, but its safety is not established in these settings. Here, we evaluated the safety and efficacy of a fixed dose of 1400 mg sqR in combination with standard-of-care chemotherapy in Ugandan adults with CD20+ DLBCL.
Methods: This open-label phase I study, conducted at the Uganda Cancer Institute [(UCI) Kampala, Uganda], enrolled patients age ≥ 18 with newly diagnosed CD20+ DLBCL with measurable disease, and an ECOG performance status of < 2. Patients living with HIV were eligible if they were receiving antiretroviral therapy and had CD4+ T-cell count >200 cells/uL. The first cohort (n=6) received IV rituximab, 375mg/m 2 on day 1 of cycle 1 plus CHOP chemotherapy (IV cyclophosphamide 750 mg/m 2, IV doxorubicin 50 mg/m 2, IV vincristine 1·4 mg/m 2 (maximum 2 mg/m 2) on day 1, and oral prednisone 100 mg on days 1-5). This cohort received sqR (1400 mg) for subsequent cycles. The second cohort (n=12) received sqR plus CHOP for all cycles. Safety and tolerability were evaluated by monitoring adverse events and graded by Common Terminology Criteria for Adverse Events (CTCAE) version 5; secondary outcomes included response rates and treatment completion. We used Kaplan-Meier methods to estimate OS and progression-free survival (PFS), defined as disease progression or death, with 95% confidence intervals (95% CIs) at 12 months from initiation of treatment.
Results: Between October 25 th, 2019 and October 7, 2022, 38 patients were assessed for eligibility; 19 patients met the criteria and were enrolled. One patient was not given the study treatment due to UCI-mandated COVID-19 restrictions. Of the 18 participants, median age was 36.5 years (IQR 25.2-50.0), 10 (56%) were male and 10 (56%) presented with advanced stage (III/IV) disease. Three (16.7%) participants were living with HIV and their median CD4 count was 311. Participants completed a median of 6 cycles of therapy. Grade 3 or 4 hematologic toxicities were reported in 11 (61%) participants; grade 3 or 4 non-hematologic toxicities were reported in 4 (22%). The most common hematologic toxicity was neutropenia (n=9, 50%); the most common non-hematologic toxicities were anorexia (n=7; 39%) and hyponatremia (n=7; 39%).
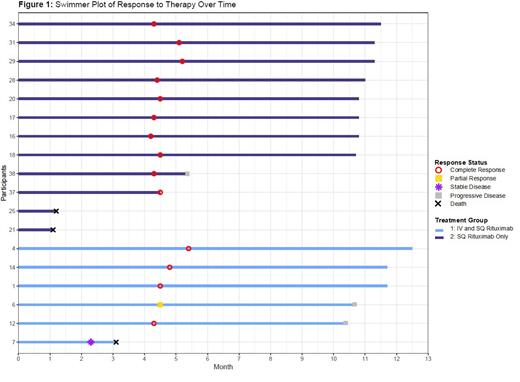
Fifteen of the 18 participants completed treatment; 3 died during treatment. Of the 15 who completed treatment, 14 (93.3%) patients achieved a complete response (CR) and 1 patient (6.7%) had a partial response (PR). The patient who had a PR subsequently developed progressive disease, as did 2 patients who initially had a CR after therapy (Figure 1). The OS and PFS at 12 months was 83% (95% CI, 68-100%) and 66% (95% CI 47-92%) respectively.
Conclusion: As demonstrated in other parts of the world, sqR together with CHOP chemotherapy was safe, well-tolerated, and efficacious among Ugandan patients with DLBCL. The very high CR, PFS, and OS rates are nearly double that of historical controls at the UCI, and comparable to outcomes expected in resource-rich settings. Colleagues have previously demonstrated the safety and efficacy of an oral chemotherapy regimen for aggressive non-Hodgkin's lymphoma in East Africa (Mwanda et al. [PMID: 19470940]). Given the results of this study, the potential for a safe and effective regimen, which precludes the need for IV therapy, for the treatment of DLBCL exists. Additionally, this trial strengthened the clinical trial research infrastructure in Uganda; trials conducted locally are critical to determining pragmatic and optimal treatment strategies. We anticipate that this research will improve care for DLBCL patients not only in Uganda but in other resource-limited settings as well.
Disclosures
Uldrick:Regeneron: Current Employment. Menon:GSK Open Africa Lab: Research Funding; Roche: Research Funding; Cepheid Diagnostics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal